By Rashmi Acharya, MS; Andrew Ciupek, PhD; Jennifer C. King, PhD; Amy C. Moore, PhD; Daniel Saez, MS; Jacinta Wiens, PhD
This past weekend was the ASCO Annual Meeting with presentations of some of the most important lung cancer data of the year. GO2 for Lung Cancer’s Science & Research team recaps highlights for you:
ADAURA Clinical Trial
The most talked-about data was the ADAURA clinical trial, a trial for people who had early stage EGFR-positive lung cancer – stages IB through IIIA. The results showed that taking Tagrisso (osimertinib) as an additional therapy (known as an “adjuvant” therapy) after surgery and sometimes chemotherapy made a significant difference in the percentage of patients who had a recurrence. After 2 years, 89% of patients on Tagrisso were lung cancer-free vs. 53% of those not taking it. Although it is too soon to know if the patients taking Tagrisso survived longer, the presenter called this “practice changing.” We expect that this will become an approved treatment option and recommend that eligible patients have an informed conversation with their treatment team about the positives and negatives of taking adjuvant Tagrisso. One important implication of this study is the need to have biomarker testing to inform treatment – no matter what stage the cancer is.
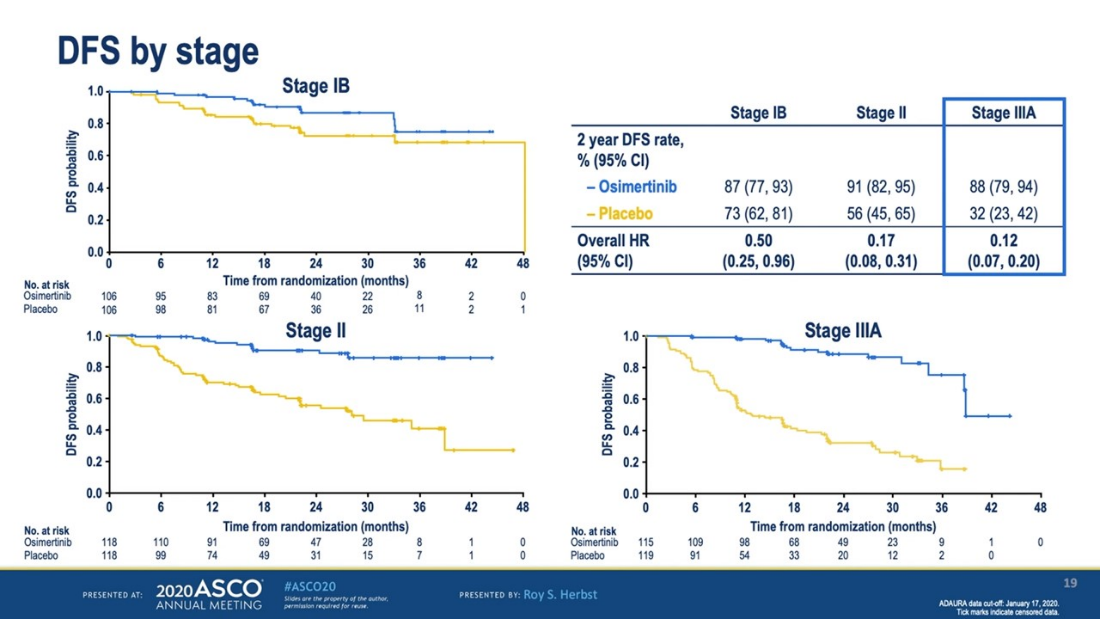
Disease Free Survival Curves for Adjuvant Tagrisso (blue) vs control (yellow), by Stage of Lung Cancer
More Advances in Targeted Therapy
In addition to ADAURA, several presentations focused on advances in targeted therapy, describing new and additional options for many different biomarker-positive lung cancers.
HER2
- Results from the Destiny-Lung01 trial showed promising effects of the new HER2 targeted therapy, Enhertu (trastuzumab deruxtecan), for patients with stage IV HER2-mutated NSCLC, even when they had already had other treatments that had stopped working. This data is extremely hopeful for patients with NSCLC with HER2 mutations as there are currently no approved targeted therapies for these patients.
MET
- Tabrecta (capmatinib), which has been recently approved by the FDA for treatment of NSCLC with MET exon 14 skipping mutation, shows potential for treatment of cancers with MET amplification in patients that have not received prior treatment within the scope of a clinical trial (GEOMETRY). Early data shows that 40% of patients with MET amplification that had not received previous treatment responded to Tabrecta. This gives a new population of patients, mainly men with a history of smoking, promise for future FDA approved treatment for MET driven NSCLC.
- New MET drugs, Sym015 and tepotinib, are currently in clinical trials for potential future treatment of patients whose cancers have MET mutations. Sym015 is being studied for patients whose cancer have both exon 14 skipping mutation and gene amplification with the potential to be used in combination with drugs like Tabrecta. Tepotinib is being studied for patients whose cancer has the exon 14 skipping mutation. These drugs give patients potential for new options in the space.
RET
- Updated data from the LIBRETTO-001 trial of the RET targeted therapy Retevmo (selpercatinib) showed that the drug was effective even for patients that had their cancer spread to the brain. Retevmo was approved to treat stage IV RET-fusion positive NSCLC early last month.
- Early data from the ARROW trial of the new RET targeted therapy pralsetinib showed that the drug had promising effects in treating RET-fusion positive NSCLC even when the cancer had spread to the brain. Pralsetinib may represent an additional treatment option for RET-fusion positive patients.
Other Targets and Resistance to Targeted Therapies
- Clinical trials are being conducted to find drugs to target a mutation associated with lung cancers having squamous cell type called PIK3CA. Results from these trials are still very early but could point to new options for cancers having squamous cell type.
- AMG510 (sotorasib) continues to have promising data in clinical trials (Code Break 100) for patients whose cancer has a KRAS G12C mutation. Phase 2 for the trial is currently underway.
- Several studies were presented that may have implications for targeted therapy resistance which remains a problem for many patients being treated with these drugs. This underscores the importance of continuing to seek biomarker testing at the point of progression to make sure your next treatment is specific to your cancer whenever possible. Studies included looking at combination therapy for EGFR-positive NSCLC and understanding MET resistance pathways.
New Ways to Use Immunotherapies
- An update from the CheckMate 227 trial showed that stage IV non-small cell lung cancer (NSCLC) patients who received the recently approved immunotherapy combination of Opdivo (nivolumab) and Yervoy (ipilimumab) as their first treatment were still living longer then patients who got chemotherapy alone. If the cancer was responding to the treatment at 6 months, most people continued to benefit for at least 3 years. This continues to show that immunotherapy combinations can lead to long term benefits for many patients.
- The CheckMate 9LA trial showed that the immunotherapy combination of Opdivo (nivolumab) and Yervoy (ipilimumab) given together with a limited chemotherapy dose as a first treatment helped patients to live longer then when they just received chemotherapy alone. This immunotherapy combination was approved as a first treatment for stage IV NSCLC last month representing another immunotherapy option for patients.
- For small cell lung cancer, the Imfinzi (durvalumab) + chemotherapy arm of the CASPIAN study showed overall survival benefit for first line treatment of extensive stage with 52.8% survival at 12 months and 22.2% at 24 months. It was approved by the FDA on March 27, 2020. Other chemotherapy + immunotherapy combination studies for small cell with Opdivo (nivolumab) and Keytruda (pembrolizumab) showed them as acceptable platforms for developing new treatment strategies but not changing current practices.
COVID-19 and Cancer
This was indeed an unprecedented year for ASCO, which was forced to hold a virtual meeting in response to the ongoing COVID-19 pandemic. The SARS-CoV-2 virus poses a unique and ongoing threat to the lung cancer community. Previous studies have reported increased mortality in lung cancer patients with COVID-19. This year’s ASCO included two relevant presentations:
- The Thoracic cancERs international coVid 19 cOLlaboraTion (TERAVOLT) is specifically tracking outcomes for lung cancer patients infected with COVID-19. At AACR, TERAVOLT reported early data showing a 34.6% mortality rate in lung cancer patients with COVID-19. The ASCO update included 400 patients, the majority of which had stage IV cancer. Patients receiving chemotherapy, either alone or in combination, within three months of a diagnosis of COVID-19 fared the worst, with a significantly increased risk of dying (64%) compared to those who did not receive chemotherapy.
- By contrast, the COVID-19 and Cancer Consortium (CCC19) registry is tracking outcomes across all cancer types. The major finding from this study is that patients with actively progressing cancer were five times more likely to die within 30 days of diagnosis with COVID-19 compared to patients who were in remission or had no evidence of disease. In a study of over 900 patients, the death rate for cancer patients was 13%, nearly double that of all patients with COVID-19.
The key takeaway is that cancer patients are especially vulnerable to worse outcomes from COVID-19.
Survivorship
- Multiple studies with comprehensive geriatric assessment and intervention recommendations by geriatricians incorporated into cancer care showed improved quality of life, reduced hospitalization and treatment discontinuations. These study results demonstrate that cancer care delivery can be improved with an integrated care model in the elderly patient group and should be implemented as standard of care.
- Clinical trials with acupuncture showed it reduces symptoms of chemotherapy induced neuropathy (CIPN) and chronic pain in cancer patients. A phase 3 study also showed Yoga for Cancer Survivor (YOGAS) and CBT are helpful in management of insomnia. These treatments can be added as supportive treatments for symptom management of anti-cancer treatment improving overall quality of life and survivorship experience.
In summary, ASCO demonstrated that while many of us may be staying at home or working from a distance, the scientific community is still working hard to find new ways to improve detection, treatment, and survivorship of lung cancer. It has been an unprecedented time with 7 new FDA approvals for lung cancer in May alone and we are confident that research and innovation will continue to fuel advances, despite the pandemic.

Leave A Comment