As of September 18, 2020, the US has had 6.7 million cases of COVID-19, with just over 198,000 deaths. The Midwest is leading new cases, with 8 cities in Wisconsin appearing on The New York Times list of the 20 metro areas with fastest-growing cases.
With the run-up to the US Presidential election now less than two months away, recent weeks have seen a growing national dialogue on the potential availability of a SARS-CoV-2 vaccine. In this week’s update, we want to review some basic concepts on vaccines, the clinical trials process for ensuring vaccine safety and provide an update on the current status of the various vaccine candidates currently under development.
What is a vaccine? How long do vaccines last?
In the most basic terms, a vaccine is a substance that can stimulate the body’s immune response to provide protection against diseases caused by different viruses and bacteria. Some vaccines provide potentially life-long protection (measles) while others provide long-term protection but still require periodic “booster” shots (tetanus being a classic example). Still others require annual vaccination because of the nature of the virus – influenza virus (that causes “flu”) undergoes changes from year to year and so the formulation for the vaccine changes each year to accommodate these changes and offer the best protection possible.
(PSA: don’t forget to get your flu shot this year!)
How are vaccines tested?
Everyone feels a great sense of urgency to develop a vaccine for SARS-CoV-2 so we can think about returning to some degree of “normalcy” in our daily lives. A concerted global effort is currently underway not only to develop a safe and effective vaccine but to develop other treatments as well (including so called monoclonal antibodies as well as novel antiviral treatments). In the US, the administration has developed what it refers to as “Operation Warp Speed” to try to accelerate vaccine development.
Without getting into a political debate, we want to offer a brief overview of what goes into getting a vaccine approved. Specifically, once a candidate vaccine is identified, its safety and efficacy (how well it works) must be validated through a rigorous clinical trials process as shown in the schematic below:
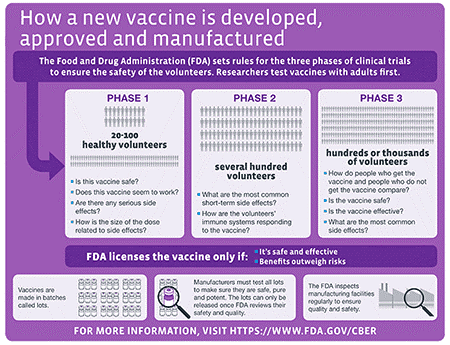
For a great overview of how vaccines are developed, the different types of vaccines, how they are tested and the status of current efforts to develop a SARS-CoV-2 vaccine, we refer you to an excellent resource put together by The New York Times.
Vaccine Safety
Historically, the United States Food and Drug Administration’s Center for Biologics Evaluation and Research (CBER) has been responsible for regulating vaccines in the US. Recently, the scientific integrity of both the FDA and the Centers for Disease Control and Prevention (CDC) have come into question over fears that they may be rushing vaccine development in the interest of political expediency. Because of this concern, many of the pharmaceutical companies at the forefront of the effort to develop a SARS-CoV-2 vaccine signed an unprecedented pledge affirming their commitment to vaccine safety.
Politics aside, the scientific community must ensure any potential vaccine is both safe AND effective before it is approved and administered to the public. Past experience with the development of SARS and MERS (Middle-Eastern Respiratory Syndrome) vaccines has taught us that coronavirus vaccines need thorough testing. A recent incident that occurred during the Phase 3 clinical trial of AstraZeneca’s vaccine candidate highlights why vaccine safety is paramount. The initial lack of details about the nature of the incident raised concerns about lack of transparency by the drug companies developing these vaccines. In response to mounting pressure, several of the leading contenders have made their protocols public.
Hope on the Horizon
Despite the challenges associated with developing an effective vaccine against SARS-CoV-2, there are several reasons to be hopeful:
- The science is advancing at a historic and unprecedented pace. Previously, the fastest vaccine ever made (against mumps) took four years to develop.
- We have access to novel vaccine development platforms and also experience with coronavirus vaccine development with SARS and MERS. Scientists are building on this pool of available knowledge to develop a vaccine against SARS-CoV-2.
- We have gone from first identifying a novel virus (SARS-CoV-2) as the cause of COVID-19 (Dec 2019) to having the sequence of the viral genome (Jan 2020) and the pursuit of multiple, compelling vaccine efforts within the span of only six months.
Resources and websites
- IASLC’s Guide to COVID-19 and Lung Cancer
- The National Cancer Institute has a special website for COVID-19 and emergency preparedness. COVID-19: What People with Cancer Should Know
- We are following updates provided by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC)
- Johns Hopkins COVID-19 Resource Center
- Interactive map of US COVID-19 cases by state
- The One-Two Punch: Cancer And COVID-19 (an important perspective for cancer patients)
- You can find information specific to your state or city or town on your health department’s website.
- American Medical Association resources for healthcare providers.





GO2 for Lung Cancer (Amy Moore, PhD – amoore@go2.org)
LUNGevity Foundation (Upal Basu Roy, PhD, MPH – ubasuroy@lungevity.org)
Lung Cancer Foundation of America (Kim Norris – KNorris@lcfamerica.org)
Lung Cancer Research Foundation (Jan Baranski, PhD – jbaranski@lcrf.org)
LungCAN (Kimberly Lester – kimberly@lungcan.org)

Leave A Comment