The authors of this weekly advocacy update are all scientists (nerds) and so before we get to this week’s update, indulge a little humor: “May the Fourth be with you!” Now back to our regularly scheduled programming:
As of May 2, 2020, the Centers for Disease Control and Prevention (CDC) reports 1,062,446 cases of COVID-19 and 62,406 COVID-19-associated deaths. Many states are beginning to loosen restrictions and reopen certain businesses. It is worth noting that in Georgia, the first state to reopen, the shelter in place policy has been extended through June 12, 2020 for the most at-risk populations, including those over age 65, those in nursing homes or long-term care facilities, those with chronic lung disease, moderate to severe asthma, severe heart disease, class III/severe obesity, diabetes, liver disease, chronic kidney disease and undergoing dialysis, as well as those who are immunocompromised.
On Friday, May 1, 2020, the Food and Drug Administration (FDA) granted emergency use authorization for the antiviral drug, remdesivir, for the most severely ill COVID-19 patients. While this is an encouraging development, we must provide a word of caution in that we still do not have effective treatments for broad use by the general public or a vaccine.
In light of the ongoing COVID-19 pandemic, the American Association of Cancer Research (AACR) shifted its annual meeting to a virtual format and broke it into two separate events. The first was held on April 27 -28, 2020. In this week’s update, we will discuss some of the information presented during this meeting and what it means for the cancer community. In particular, we will focus on answering three questions:
- What are the implications of COVID-19 for my personal cancer treatment?
- How do I make sense of contradictory COVID-19 information?
- What are the impacts of COVID-19 on cancer research?
What are the implications of COVID-19 for my personal cancer treatment?
Since we first started providing these updates in early March 2020, there has been growing evidence that lung cancer patients infected with COVID-19 have worse outcomes. During the AACR plenary session on “COVID-19 and Cancer,” an international team led by Dr. Marina Garassino presented early data for TERAVOLT, a global registry collecting characteristics and outcomes of patients with thoracic cancers affected by COVID-19. They reported a disturbingly high mortality rate of 34.6% (66/191) among patients with thoracic cancers.
As research advocates serving the lung cancer community, we recognize that these data are alarming. The immediate implications of these results fall in line with what we have been advising those who fall in high-risk groups, including lung cancer patients: continue to practice social distancing when possible, wear protective face coverings when out in public, wash hands often, and minimize travel to essential needs only (medical appointments, procuring groceries or prescriptions).
Our April 20, 2020 update discussed the increasing role for telehealth in management of patient care and our April 27, 2020 update focused on the guidelines issued by leading medical organizations and societies. Our March 30, 2020 update included impacts on clinical trials – as states begin to reopen, some trial sites are resuming enrollment. Thus, it remains imperative that you talk with your treatment team about your individual treatment plan.
Indeed, as a result of the COVID-19 pandemic, doctors and scientists are reevaluating treatment schedules and the “usual way of doing business.” One example is the recent April 28, 2020 FDA approval for a new dosing regimen for the immunotherapy drug, pembrolizumab. This approval is based on data presented at the 2020 AACR Virtual Meeting.
Dr. Jacob Sands, a leading lung cancer medical oncologist at Dana-Farber Cancer Institute, provides a nice discussion on the importance of individualized lung cancer management in the COVID-19 era here.
How do I make sense of contradictory COVID-19 information?
We recommend that you follow information from trusted and medically vetted websites such as the CDC, the WHO, and the IASLC. Information on how to access these websites are included in the Resources and websites section below.
Our understanding of COVID-19 is evolving rapidly. This means that what was true a month ago may not be true under current circumstances, as doctors and scientists generate more evidence. You might hear contradictory information from different sources or at different times. As an example, the anti-malarial and autoimmune disease drug, hydroxychloroquine, was shown to have positive effect in COVID-19 patients in early studies. However, further study with more patients showed hydroxychloroquine was not as effective as it was initially thought to be, and highlighted the fact that hydroxychloroquine comes with a range of side effects that make it unsuitable for use in patients with heart issues. This is a great example of the scientific method whereby a finding or a hypothesis changes as new information is gathered.
We also caution on how one should interpret information shared across media and press during these times. COVID-19 is a global pandemic and is affecting the oncology community everywhere in the world. Given the urgent global need for information on effective COVID-19 management, healthcare providers are sharing preliminary information as quickly as possible with the goal of learning from each other’s experiences. This means that not all information shared publicly will have the same level of evidence as formal clinical trials. The information is important and valuable, but it is not yet validated in large groups of patients.
When judging what you read from publicly available sources, we suggest you use the Evidence-Based Medicine (EBM) Pyramid as a guiding framework.
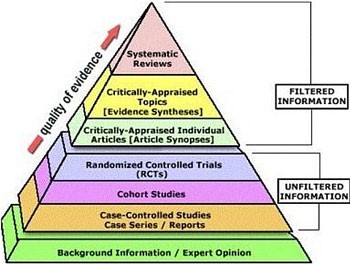
EBM Pyramid and EBM Page Generator, copyright 2006 Trustees of Dartmouth College and Yale University.
All Rights Reserved. Produced by Jan Glover, David Izzo, Karen Odato and Lei Wang.
Higher quality of evidence takes longer to get published to allow for collecting larger amounts of data, statistical analysis, and scientific peer review. Most of the literature currently available on COVID-19 and lung cancer are case reports and studies of a small number of patients in a few institutions. COVID-19 has not been around long enough to enable large, formal clinical trials about its impact on lung cancer treatment. If you have questions about whether published COVID-19 findings might affect your lung cancer treatment, please discuss your individual situation with your treating physician.
What are the impacts of COVID-19 on the state of academic cancer research?
Most academic research institutions, including universities and hospitals, have shut down most research labs and closed enrollment in some clinical trials to accommodate government-imposed shelter in place mandates and protect researchers’ lives. Only critical research, such as maintaining cell lines or animal models for preclinical research and some clinical trials with strong evidence of effectiveness, is being allowed to continue. These are institutionally mandated restrictions that have been put into place to protect university staff. Some researchers who are also clinicians have been deployed to assist with COVID-19-related clinical duties. Bench scientists who are not involved with clinical duties have been advised to work from home in activities such as grant and manuscript writing, and data analysis.
Funders of academic lung cancer research in the US such as the National Cancer Institute, the Department of Defense, and private non-profits (e.g., LUNGevity Foundation, GO2 for Lung Cancer, Lung Cancer Research Foundation, Lung Cancer Foundation of America) have all made concessions to accommodate the needs of the scientific community and best support investigators during this critical time, while trying to minimize any delays in lung cancer research. Concessions include:
- Extended deadlines for grant applications
- Allowing the use of grant funds for salaries and stipends even when researchers are not working in the laboratory
- Flexibility regarding project extensions and accommodating unanticipated costs such as loss of animals and chemicals bought for experiments
- Allowing grantees more time to report on awards after an award is completed
- Numerous flexibilities regarding expenditures of funds, such as money already spent in conferences and travels





GO2 for Lung Cancer (Amy Moore, PhD – amoore@go2.org)
LUNGevity Foundation (Upal Basu Roy, PhD, MPH – ubasuroy@lungevity.org)
Lung Cancer Foundation of America (Kim Norris – KNorris@lcfamerica.org)
Lung Cancer Research Foundation (Jan Baranski, PhD – jbaranski@lcrf.org)
LungCAN (Kimberly Lester – kimberly@lungcan.org)

Leave A Comment