By Jennifer C. King, Ph.D., Chief Scientific Officer, GO2 for Lung Cancer
Are scientists learning enough about how people just like you respond to new treatments? You may be surprised. Clinical trial participation rates among people with cancer in the United States are low, with only about 8% of those diagnosed joining a trial1. Unfortunately, it is hard to learn about treatments and their effects in a normal healthcare setting because it is difficult to combine data from different doctors and healthcare systems. That means that data from clinical trials provide the most complete information about how new treatments work and for whom.
Where this becomes a problem is when you look at who is participating in clinical trials. Those who enroll are generally younger and healthier than the average patient. Among people with cancer in the U.S. that participate in clinical trials, only 6% are Black, 3% are Asian American, and 2% are Hispanic2. This low participation by communities of color holds true for lung cancer trials, where Black people comprise 15% of participants and Asian/Pacific Islanders and Hispanics comprise less than 4% each3.
This can have significant consequences. A 2019 analysis showed that Black and Hispanic groups are “consistently underrepresented” in landmark oncology trials that lead to FDA drug approvals4. Representation Matters. Treatments can work differently in people. So, it is important to understand how all people of all ages and racial and ethnic groups respond to them. Given that lung cancer is the leading cause of cancer deaths in the United States, representative participation of racial and ethnic minorities in lung cancer clinical trials has the potential to significantly improve cancer disparities5.
We encourage everyone diagnosed with lung cancer to speak with their treatment team about whether a clinical trial may be an appropriate treatment choice. GO2 for Lung Cancer’s LungMATCH specialists can also help identify clinical trials that may be right for you. It is important that people from all communities enroll in trials so that we can learn how new treatments work for people of any age, gender, race, and ethnicity.
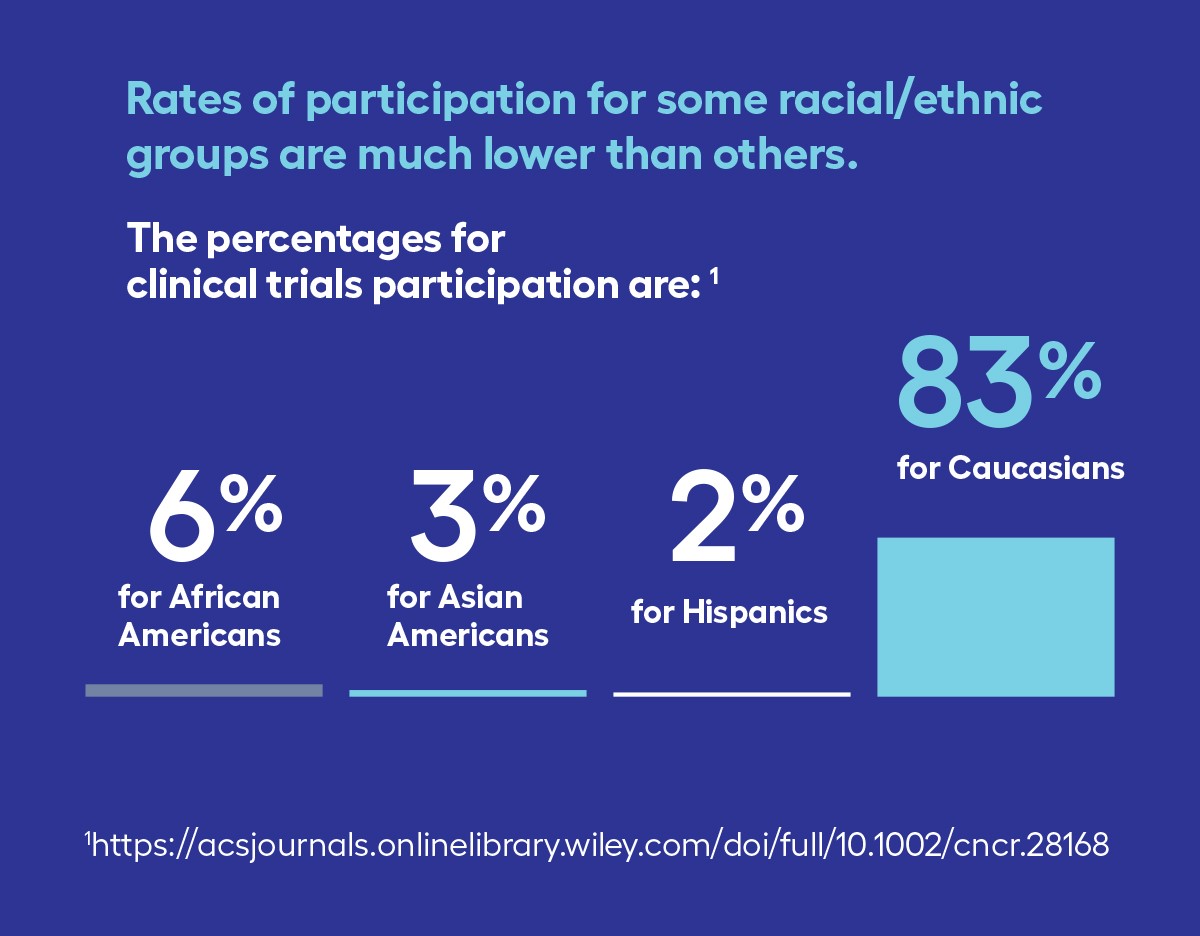
1 Unger, J. M., Vaidya, R., Hershman, D. L., Minasian, L. M., & Fleury, M. E. (2019). Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. JNCI: Journal of the National Cancer Institute, 111(3), 245-255.
2 Kwiatkowski, K., Coe, K., Bailar, J. C., & Swanson, G. M. (2013). Inclusion of minorities and women in cancer clinical trials, a decade later: have we improved?. Cancer, 119(16), 2956-2963.
3 Pang, H. H., Wang, X., Stinchcombe, T. E., Wong, M. L., Cheng, P., Ganti, A. K., … & Redman, M. W. (2016). Enrollment trends and disparity among patients with lung cancer in national clinical trials, 1990 to 2012. Journal of Clinical Oncology, 34(33), 3992.
4 Loree, J. M., Anand, S., Dasari, A., Unger, J. M., Gothwal, A., Ellis, L. M., … & Raghav, K. (2019). Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol 5: e191870–e191870.
5 Zullig, L. L., Sims, K. J., McNeil, R., Williams, C. D., Jackson, G. L., Provenzale, D., & Kelley, M. J. (2017). Cancer incidence among patients of the US Veterans Affairs Health Care System: 2010 update. Military medicine, 182(7), e1883-e1891.

I think older people are being left out of clinical trials simply because of their age. There are a lot of “older people” who are healthy enough to be considered.
It’s important to know that the underrepresentation of Black and Hispanic groups in significant oncology trials, as revealed by a 2019 analysis, highlights the importance of diverse participation in research. Representation matters greatly when it comes to healthcare, as individuals from different backgrounds may respond to treatments in unique ways. I believe though that recognizing and addressing these disparities can lead to more effective and tailored healthcare for all, ensuring equitable access to treatments and improving health outcomes for marginalized communities.